Study overview
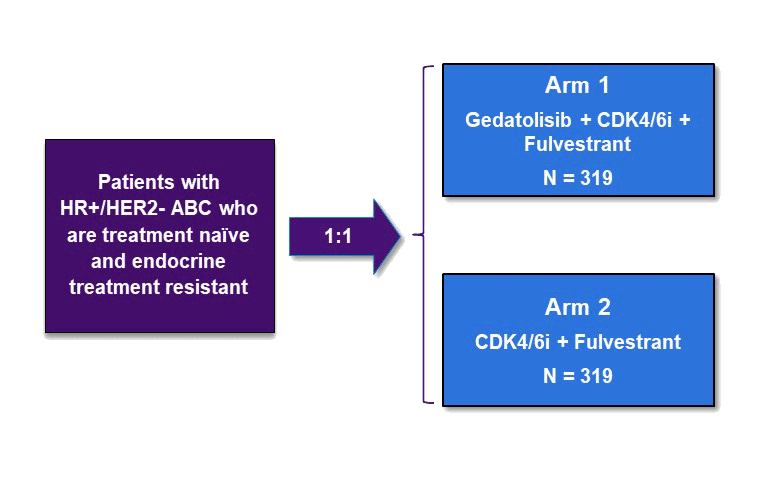
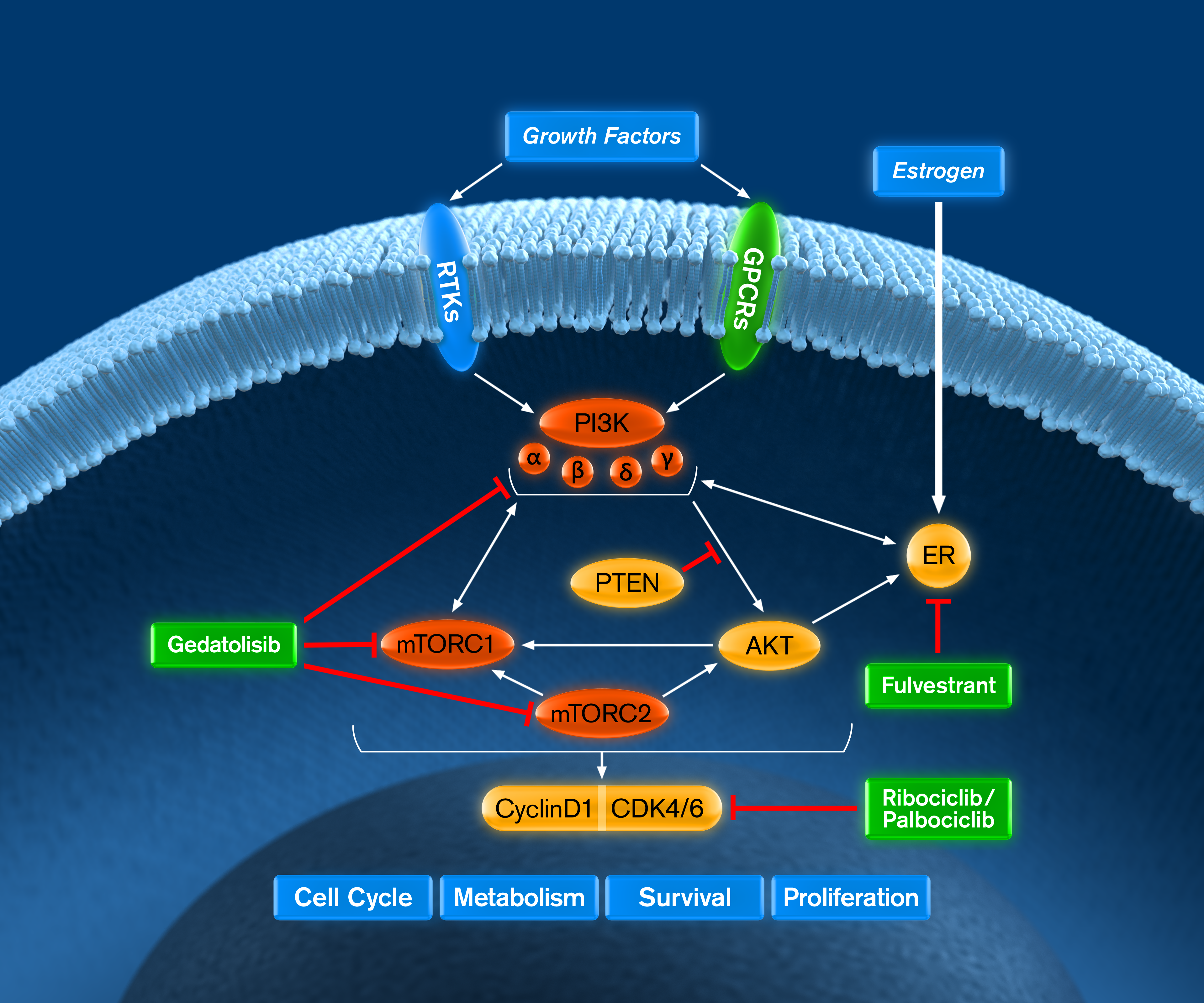
VIKTORIA-2 is a Phase 3 clinical trial evaluating gedatolisib plus a CDK4/6 inhibitor and fulvestrant as first-line treatment for patients with HR+/HER2- advanced breast cancer (ABC) who are endocrine therapy resistant. For the CDK4/6 inhibitor, investigators may choose either ribociclib or palbociclib.
Approximately 638 eligible patients whose PIK3CA mutational status has been determined will be enrolled.
Patients will be assigned to a cohort based on the PIK3CA status of their tumor (MT or WT) and then randomized on a 1:1 basis to receive either the investigational regimen of gedatolisib, a CDK4/6 inhibitor and fulvestrant (Arm 1) or the control regimen of a CDK4/6 inhibitor and fulvestrant (Arm 2).
Prior to initiating the Phase 3 study, a safety run-in of approximately 12-36 subjects will evaluate the safety profile of gedatolisib combined with ribociclib and fulvestrant. The safety profile of gedatolisib combined with fulvestrant and palbociclib has previously been characterized.