The PI3K-mTOR Pathway
How and why we target the PI3K and mTOR pathway in cancer
Gedatolisib is an investigational, intravenously administered pan-class I isoform PI3K and mTOR inhibitor with low nanomolar potency for the p110α, p110β, p110γ, and p110δ isoforms and mTORC1 and mTORC2. Its mechanism of action and pharmacokinetic properties are highly differentiated from other currently approved and investigational therapies that target PI3K or mTOR alone or together. We believe this will enable gedatolisib to treat a broader patient population than inhibitors that only target PI3K or mTOR.
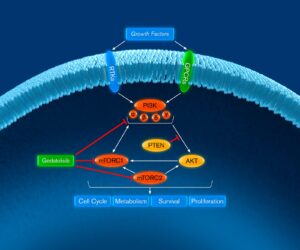
It has been reported that inhibition of one PI3K isoform may be offset by the increased activity of the other isoforms through different adaptive mechanisms. Inhibiting all four PI3K isoforms, as gedatolisib does, may thus prevent the confounding effect of isoform interaction that may occur with isoform-specific PI3K inhibitors.
Each PI3K isoform is known to preferentially affect different signal transduction events that involve tumor cell survival, depending upon the aberrations associated with the linked pathway. A pan-PI3K inhibitor can thus treat tumors harboring abnormalities that signal through the different PI3K isoforms, which would potentially induce anti-tumor activity in a broader population of patients than an isoform-specific PI3K inhibitor.
As a potent inhibitor of mTORC1 and mTORC2, in addition to PI3K, gedatolisib inhibits the PI3K/AKT/mTOR pathway both upstream and downstream of AKT. Since inhibiting mTOR can also relieve normal inhibitory mechanisms resulting in activation of the PI3K pathway, there is a compelling rationale for simultaneous inhibition of PI3K and mTOR.

How and why we target the PI3K and mTOR pathway in cancer

We offer access to investigational medicines