Study overview
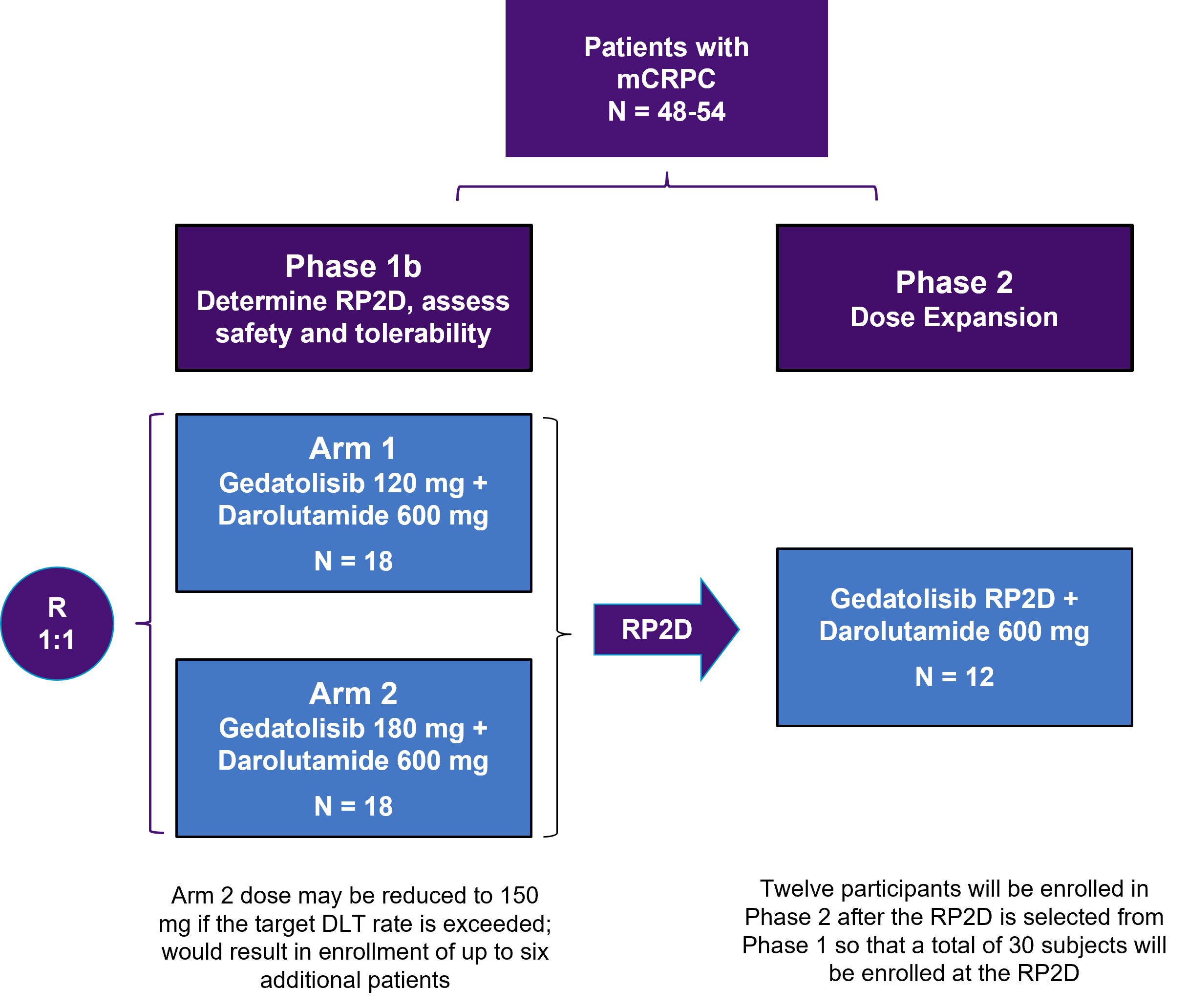
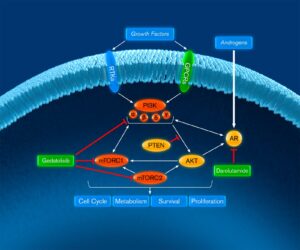
The CELC-G-201 Phase 1/2 clinical trial is evaluating gedatolisib plus darolutamide, an androgen receptor (AR) inhibitor, in patients previously treated with an AR inhibitor for metastatic castration resistant prostate cancer (mCRPC).
Approximately 48 to 54 eligible patients will be enrolled.
In the Phase 1 portion of the study, 18 participants will be randomly assigned to each of 2 dose arms (n = 36 total) and evaluated in two parts. Arm 1 will evaluate 120 mg gedatolisib, and Arm 2 will evaluate 180 mg gedatolisib.
In the Phase 2 study, an additional 12 participants will then be enrolled at the recommended Phase 2 dose (RP2D) level. The total number of participants will be approximately 48 to 54, with 30 participants enrolled at the RP2D level.