Pursuing first-in-class therapies targeting the PAM (PI3K/AKT/mTOR) pathway
The PI3K/AKT/mTOR pathway has been implicated in breast, prostate, endometrial, ovarian, and hematological cancers, among others. We are currently developing our lead drug candidate, gedatolisib, a PAM pathway inhibitor, to treat advanced breast cancer and metastatic castration resistant prostate cancer.
Gedatolisib pipeline
The safety and efficacy of investigational agents and/or investigational use of approved products have not been established. Ibrance® is a registered trademark of Pfizer, Inc. Nubeqa® is a registered trademark of Bayer AG. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PIK3CA, phosphatidylinositol-4,5-biphosphate 3-kinase catalytic subunit alpha; MT, mutated; HNSCC, head and neck squamous cell carcinoma; SCLC, squamous cell lung cancer; mCRPC, metastatic castration resistant prostate cancer.
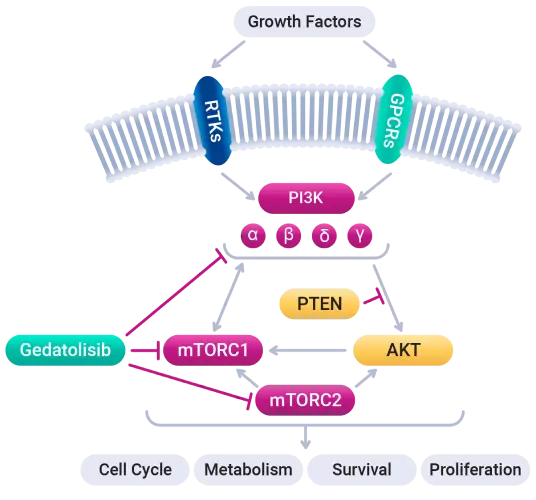
Our lead drug candidate, gedatolisib, offers a new approach to inhibiting the PAM (PI3K/AKT/mTOR) pathway
To overcome the challenges of inhibiting the PAM pathway, we are developing a potential first-in-class multi-target PAM inhibitor that:
- Targets all Class I PI3K isoforms and mTORC1 and mTORC2
- Offers a favorable pharmacokinetic profile designed to limit the toxicities typically associated with single-target PI3K, AKT, or mTOR inhibitors
Gedatolisib is an investigational pan-class I isoform PI3K and mTOR inhibitor with low nanomolar potency for the p110α, p110β, p110γ, and p110δ PI3K isoforms, as well as mTORC1 and mTORC2, that induces comprehensive blockade of the PAM pathway. Its mechanism of action and pharmacokinetic properties are highly differentiated from other currently approved and investigational therapies that target PI3Kα, AKT, or mTORC1 alone. We believe this will enable gedatolisib to treat a broader patient population than these single-target inhibitors
Ex vivo studies found that gedatolisib inhibited higher levels of PI3K/AKT/mTOR involved signaling activity than single target PI3K, AKT, or mTOR therapies, regardless of PIK3CA mutational status. Superior drug synergy when combined with other targeted therapies has also been demonstrated.
Initial clinical development is focused on breast and prostate cancers
Gedatolisib’s initial clinical development program is focused on the treatment of patients with estrogen receptor positive (ER+), human epidermal growth factor receptor 2 -negative (HER2-), advanced or metastatic breast cancer and patients with metastatic castration resistant prostate cancer. Unlike PI3K or AKT therapies that are only approved to treat patients with PIK3CA or PTEN mutations, gedatolisib is under development for patients with and without PIK3CA mutations.
Learn about VIKTORIA-1, evaluating 2nd line treatment in advanced breast cancer
Discover VIKTORIA-2, evaluating 1st line treatment in advanced breast cancer