The CELsignia platform identifies patients with abnormal oncogenic signaling pathways to detect new treatment options for patients.
Celcuity was founded to develop a better way to diagnose and treat cancer. For roughly 20% of cancer patients, a molecular test can identify their cancer driver. For the other 80% of cancer patients, their cancer driver is undiagnosed, which may preclude patients from receiving the optimal drug to treat their cancer.
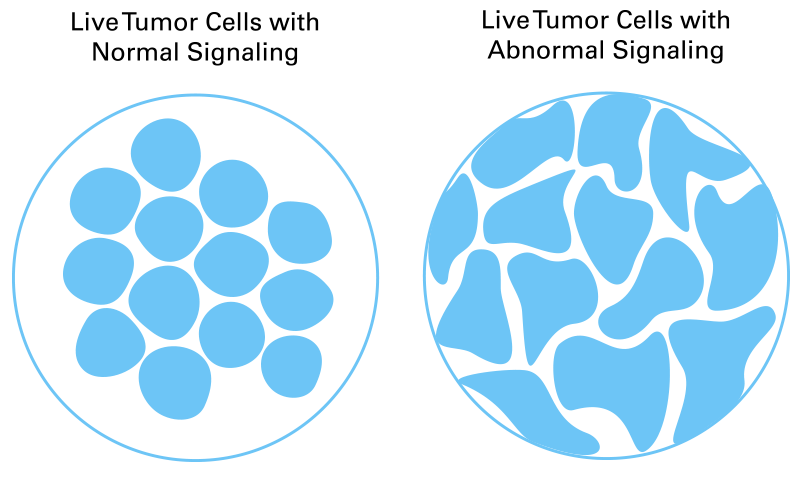
CELsignia uses a patient’s own live tumor cells to measure pathway signaling, the activity that drives cancer. Abnormal signaling is not detected by genomic tests but looks distinctly different from normal cellular activity, allowing CELsignia to identify cancer drivers that other tests miss.
Patients diagnosed with an abnormal pathway signaling via the CELsignia platform may benefit from a targeted therapy designed to block the activity of that pathway.